As featured in waterline Autumn 2019
CORROSION IN CLOSED HEATING AND CHILLED WATER SYSTEMS
– CAUSES AND PREVENTION
Phillip Munn
Midland Corrosion Services Ltd.
A paper containing useful information from the WMSoc Archive. Many of our papers are still relevant some years after being published.
Anyone wishing to add information should write to the Waterline editorial team.
First published: October 2013.
1. Introduction
Modern large buildings, including hospitals and office blocks, rely on low temperature hot water (LTHW) and chilled water (CHW) systems to provide air-conditioning. Chilled water systems also provide critical cooling for data centres housing large numbers of servers. Anyone who has been involved in the installation, commissioning and running of these systems including water treatment companies are well aware of the potential for corrosion. The resulting costs involved in damages to pipework and system components, disruption to businesses and possible legal expenses can often run into hundreds of thousands if not millions of pounds.
Later this year, the BSRIA Guide BG50 ‘Water treatment of closed heating and cooling systems’ is due to be published. This not only covers water treatment of these systems but also discusses how corrosion is caused and what steps should be taken at design, installation, commissioning and maintenance to minimise the likelihood of corrosion occurring. BS EN 14868 – ‘Guidance on the assessment of corrosion likelihood in closed water recirculating systems’ is also a useful document.
2. Corrosion of Materials
Virtually all metals (with the exception of noble metals like platinum and gold) can undergo corrosion in aqueous environments. In LTHW and CHW systems, carbon steels, copper and copper alloys, aluminium, galvanised steel and stainless steels can suffer either general corrosion and/or localised corrosion, such as pitting and stress corrosion cracking. The corrosion can manifest itself in primary corrosion damage, such as leakage of the system or erosion corrosion of control valves where failures occur as a direct consequence of corrosion of the component or pipework. However, probably more common is secondary corrosion damage due to the production of corrosion products resulting in blockages and circulation problems elsewhere in the system.
Table 1, taken from BG50, lists the different alloy types and non-metals, where they are used in systems, and the different forms of corrosion or degradation to which they may suffer.
Table 1 – Different alloys and non-metallic materials and their corrosion resistance
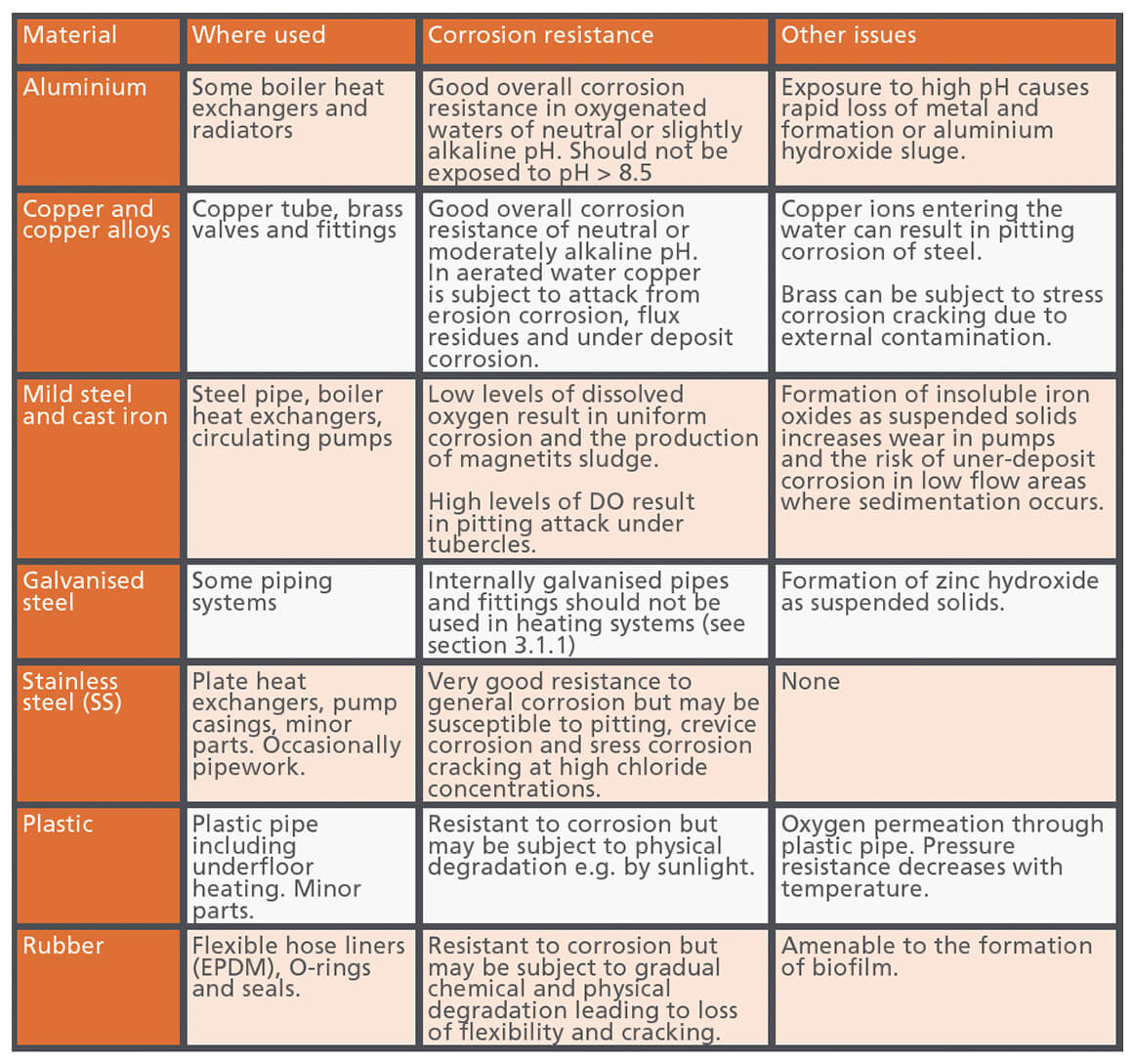
Stress corrosion cracking initiating from pit in austenitic stainless steel

There are many factors which can influence whether corrosion of a particular material will take place and the rate of corrosion if it does occur. These are:
- • Aeration – dissolved O2 in water
- • Water composition – hardness, chlorides, sulphates, pH, conductivity
- • Temperature
- • Flow
- • Deposits/flux residues
- • Bimetallic couples – esp. Cu/Al
- • Water treatment – corrosion inhibitors
- • Component design / manufacture leading to creation of crevices
Of all the above factors, dissolved oxygen is the most important. This is because the reduction of dissolved oxygen in the water is the primary cathodic reaction which controls the rate of the anodic metal dissolution reactions. Therefore, reducing dissolved oxygen to virtually zero should mean corrosion rates are reduced to rates where damages will not occur. In addition, galvanic corrosion of metals such as steel and aluminium when coupled to copper cannot occur in the absence of dissolved oxygen. Where one comes across a multiplicity of failures in systems, which may include failed boilers or chillers, blocked pipework and control valves in fan coil units, the cause is invariably due to gross aeration of the system. There are a variety of ways that dissolved oxygen can enter into a system, e.g. due to failures in design, installation, commissioning and maintenance which are discussed in BS EN 14868 and in the sections below.
Some materials are prone to localised corrosion in certain environments. For instance, austenitic stainless steels and aluminium can undergo pitting attack in waters containing high chloride provided some oxygen is present. High levels of chloride ions can also arise due to the excessive use of soldering flux or the failure to flush these from the system. Pitting of carbon steel under corrosion tubercles will also occur in untreated highly oxygenated water.
While the corrosion of most materials is reduced as the pH increases, e.g. steel passivates at pH>9.5, aluminium suffers from corrosion in both acidic and alkaline conditions. At a pH>8.5, the passive aluminium oxide layer begins to break down with the formation of the soluble aluminate ion. When the pH exceeds around 9, pitting of aluminium often results.
While corrosion rates generally increase with temperature in open systems with the corrosion rate of steel approximately doubling for a 30˚C rise in temperature, MTHW systems can operate quite happily at temperatures over 100˚C due to the very low levels of dissolved oxygen in the water. However, temperature is a key factor which influences the growth of micro-organisms in closed systems with moderate temperatures between 25-45˚C being the worst.
Flow affects corrosion in a number of ways. Under moderate flow conditions, corrosion is usually minimised as passivation or formation of protective layers on metals is encouraged and inhibitor species adsorb on the metal surfaces. However, under very low flow conditions, suspended solids may deposit out on metal surfaces leading to potential blockages or under deposit corrosion with possible microbial influenced corrosion occurring. On the other hand, very high flow rates can lead to erosion corrosion or cavitation, especially at control valves. The difference between erosion corrosion and cavitation is that the former usually requires there to be some dissolved oxygen in the water while cavitation may occur due to the formation of steam bubbles in completely deaerated water.
Corrosion of steel butterfly valve due to system aeration

3. Microbial Influenced Corrosion
Microbial influenced corrosion (MIC) can affect virtually all metals. It is important to realise that, as with all forms of aqueous corrosion, MIC occurs via an electrochemical mechanism and that the microbes influence (usually increase) the rate of these reactions. Microbes enter with the filling water and, if not controlled, can rapidly multiply in closed systems doubling every 20 hours. Bacteria can circulate freely in the system water as “planktonic” bacteria or adhere to the pipe walls as “sessile” bacteria. Sessile bacteria may form colonies with protective polysaccharide coatings, known as a biofilm.
There are many hundreds of different types of bacteria but these can be classified into 3 main types depending on their need for oxygen to survive and grow:
- • Aerobes (grow in the presence of oxygen), e.g. Pseudomonas, Flavobacter.
- • Facultative anaerobes (able to grow with or without oxygen) e.g. Klebsiella, E.coli.
- • Anaerobes (grow in the absence of oxygen) e.g. Sulphate reducing bacteria (SRB).
Microbes can also be classed according to the temperatures, at which they can survive and reproduce. Although there are some thermophilic microbes that can survive at temperatures above 85˚C most are killed off by 80˚C and do not reproduce above around 55-60˚C. The ideal temperature for microbes to grow is around 25-45˚C and therefore systems should not be operated at these temperatures without the presence of a biocide. It should also be realised that deposits in a system can shield bacteria from the full temperature of the water and hence MIC can sometimes occur under deposits in radiators or pipework even when the recirculating water temperature is around 80˚C.
Not all types of bacteria cause corrosion, e.g. legionella and e-coli which, although a hazard for human health, is not an issue in closed heating and cooling systems. Others, particularly pseudomonas, do not directly cause corrosion but form slimes or biofilms on pipes under which anaerobic bacteria, e.g. SRB may grow and cause corrosion. SRB can often result in localised pitting attack on mild steel or stainless steels under deposits in pipework or radiators. Often MIC can develop under corrosion products, such as iron oxides, which have formed due to the system becoming aerated at some stage.
In addition to SRB, there are many species of bacteria that oxidise iron from a soluble ferrous (Fe2+) form to an insoluble ferric (Fe3+) form. The dissolved ferrous iron could be either from the incoming water supply or the metal surface. The ferric iron produced by these bacteria can react with chloride ions to produce ferric chloride deposits that can attack stainless steel.
As well as directly causing corrosion of metals due to the formation of aggressive species and acids by their metabolism, microbes can also influence corrosion by their action on water treatment chemicals. Common in closed systems are nitrite reducing bacteria (NRB) which break down nitrites or nitrates to form ammonia. Not only does this reduce the levels of corrosion inhibitor present but the ammonia produced is aggressive to copper and copper alloys. Stress corrosion cracking of brass valves in closed systems is often the result of the action of NRB on nitrite based inhibitors.
Stress corrosion cracking in brass fitting

4. Causes and Prevention
a. Influence of Design and Construction
The design and construction of closed heating and chilled water systems can influence corrosion in a number of ways, including:
- • Selection of materials. All materials, be they metallic or non-metallic must have industry approval for use in closed systems. Direct coupling of some materials, e.g. copper with galvanised steel or aluminium should be avoided. If plastic pipework is used, these should contain a barrier to prevent oxygen diffusion.
- • Flow Rates. These should not be too high to cause turbulent flow resulting in possible erosion corrosion or cavitation at valves. Too low water velocities, which will result in deposition of solids should also be avoided.
- • Installation of sufficient expansion capacity. Failure to do this will result in water losses through pressure relief valves and consequent make-up with fresh aerated water. In addition, expansion vessels must be installed on the suction side of the main pumps to ensure that the dynamic pressure from the pumps is positive all around the system.
- • Installation of dosing pots, sufficient drain points and water sampling points. This will enable chemical cleaning of the system to be carried out prior to commissioning and water sampling to be carried out in compliance with BSRIA BG- 29:2011, as well as control of inhibitor and biocide levels.
- • Installation of sufficient automatic air vents and isolating valves. Systems should be designed with adequate number of air vents at high points of the system to remove any air and gases from the system. There should also be a sufficient number of isolating valves at strategic locations to allow inspection and/or replacement of system components without having to drain large parts of the system and expose the pipework to the atmospheric air and fresh water when refilled.
- • Thermal insulation. Insulation such as phenolic foam is used to minimise thermal losses. However, if poorly installed, water can penetrate under insulation (especially due to condensation in chilled water systems) resulting in external corrosion under the lagging.
Erosion corrosion of brass valve seat
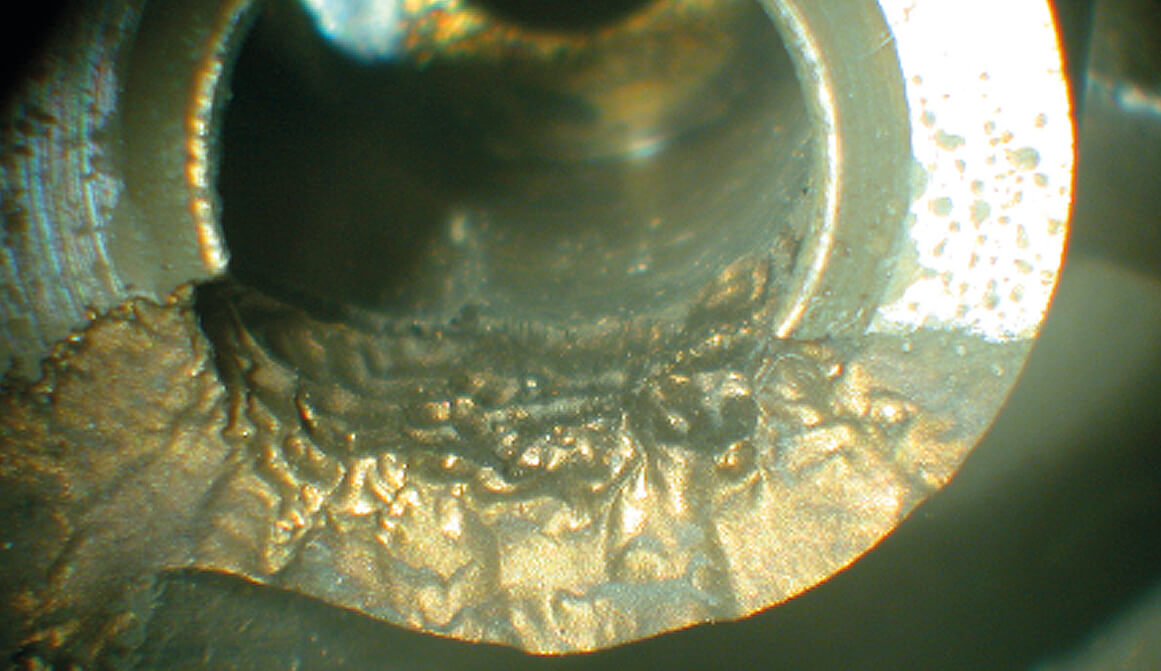
b. Influence of Commissioning and Pre-Commission Cleaning
Before commissioning, it is very important that systems are thoroughly flushed after installation to remove any debris from construction and other deposits including dirt, swarf, jointing compounds and other settled solids. Chemical cleaning can aid the removal of deposits. If left in systems, these deposits can lead to enhanced risk of MIC and pitting attack under the deposits due to differential aeration effects. They will also reduce the effectiveness of corrosion inhibitors and, if brought into circulation, can increase wear on valves and other components. BSRIA BG29/2011 is the guidance which should be followed here. Adherence to this document should also mean that the system is microbially clean at the time of hand-over by the application of a biocide after flushing and microbial analysis.
MIC occurring under debris at bottom of radiator

During the commissioning phase, the system should be correctly pressurised according to BS7074 (part 2 for LTHW and MTHW systems and part 3 for chilled water systems). Both gas pressure in the expansion vessels and pressure settings at the pressurisation unit need to be correctly adjusted. Failure to do this could mean that negative pressure at the top of the system will cause air to be sucked in leading to oxygenation of the system water. Alternatively, if pressures exceed the PRV settings, then water will be released on every heating phase and fresh aerated make-up water brought into the system. This would also dilute any inhibitors in the system.
Commissioning also involves correctly setting regulating valves to obtain the design flow rates. As discussed, too high or too low water flows can have negative consequences in terms of corrosion likelihood in the system.
In many cases, systems are altered or expanded, which involves opening up of the system. In such cases, the system should be re-commissioned and possibly re-cleaned and treated.
c. Influence of Maintenance
After handover, the responsibility for maintaining the systems and ensuring correct operation usually falls to a site engineer or separate maintenance company. Besides having to deal with unscheduled shutdowns or emergencies, they should have a list of checks that are to be undertaken every 1, 3 or 6 months. These will include checking for leaks, checking of system pressures and gas pressures in expansion vessels, monitoring inhibitor levels and generally checking and maintaining the correct operation of pumps, pressurisation units etc.
One of the main reasons why systems do not operate efficiently with blockages and failures of components occurring is that maintenance is not properly carried out. Often not enough time is given to properly carry out all of the tasks which should be performed at set intervals. This means that systems, which were initially clean, tight and well-inhibited, gradually become contaminated, aerated, or under inhibited.
d. Influence of Water Treatment
Water treatment has a long history in the UK. The use of corrosion and scale inhibitors and biocides help maintain systems in the optimum condition and should be able to cope with some aeration of the system water. However, the use of corrosion inhibitors should not be used as a substitute for mechanical means to maintain system tightness. In addition, it is important that the inhibitor level is dosed and maintained at the recommended strength. Under-dosing with anodic type inhibitors such as nitrite and molybdate can actually increase the likelihood of pitting corrosion on steel when incomplete passivation of the steel surface occurs. Over-dosing is a costly waste and has environmental consequences.
It is important that the water treatment specialist understands the system and the metals of construction. In particular, he or she needs to be aware if there is any aluminium in the system and if so the pH needs to be maintained below 8.5.
Finally, the use of inhibitors applied to dirty systems can also exacerbate problems. It is far better to clean and flush such systems and treat with a biocide before the application of a corrosion inhibitor.
5. Corrosion Monitoring
Unfortunately, despite a wealth of knowledge and experience in the UK, problems all too often arise in many systems well before the end of their expected lives. In addition, there have been numerous high profile and very expensive cases, where whole systems have had to be replaced or have required substantially remediation after only a few years of operation. In such cases, there is nearly always very little data available to be able to deduce precisely what happened and when. At most there are periodic water treatment and analysis records, which are usually incomplete.
Even regular water analysis results only provides a snap shot of the water quality every few months and, although it can give an indication that corrosion is occurring, it gives limited information as to why it is happening. Corrosion coupons are occasionally used to give the corrosion rates of the different metals in the system and these can be useful. However, these will only reveal the mean corrosion rates over the period that the coupons were installed and will not show any short-term upsets to the system operation. Corrosion monitoring by use of LPR probes (e.g. Corrator) has the advantage of giving a continual output of the corrosion rates of different metals. However, neither coupons nor LPR probes alone can explain why corrosion may be occurring. In addition, they are only usually used in the main flow and therefore would not pick up corrosion which may be occurring in remote parts of the building or under deposits.
All corrosion failures in systems should be preventable provided key parameters are continually monitored and timely intervention takes place if set limits are exceeded or if alarms are suddenly triggered. Even the use of a water meter on the make-up line to the pressurisation unit would indicate whether there were any leaks in the system before this could be picked up by water analysis. However, to date this has rarely been carried out. Other parameters which could be monitored to provide a complete picture of the health of a system are system pressures (especially at the top of the system), dissolved oxygen, pH, water conductivity and galvanic current. The use of bio probes could also be used to monitor the build-up of any biofilms in the system. To date, comprehensive monitoring of key system parameters in closed heating and cooling systems is virtually unknown. However, for larger heating and cooling systems, this may become routine in the future as comprehensive monitoring systems become available.
6. Summary
Failures in closed heating and chilled water systems due to corrosion are still all too common. The different metals in systems can undergo either general or localised corrosion depending on the metal type and the environmental conditions. The main influencing factor for corrosion in these systems is dissolved oxygen but water composition, temperature, flow, the presence of deposits and bi-metallic couples also play a role. Microbial Influenced corrosion is a particular problem under deposits or at moderate temperatures. Failures in design, installation, commissioning and maintenance can all result in system problems but adherence to good practice, in particular BSRIA guides BG29 and BG50 and BS7074, should significantly reduce the likelihood of failures. Continual monitoring of corrosion and key system components if used would mean that quick intervention to correct any faults in the system could be made before any corrosion damage occurs.












