As featured in Waterline Autumn 2022
Microbial Induced Corrosion in cooling systems and how regular monitoring can mitigate potential problems
Catherine Allen BSc Hons, Tintometer Inc.
ACKNOWLEDGMENTS
Special Thanks to Curtis Williams BSc, MSc (Hons), Chris Shaw (BSc Hons), MWMSoc), Ian Penney (BSc (Hons), FWMSoc), Kaylie Boland and Karl Hodge for their input and specialist knowledge in writing this paper.
Abstract
Microbes, as with all lifeforms, have optimal conditions in which they thrive. Unfortunately, for water treatment specialists, these conditions are just what we find in open and closed water recirculating systems. Microbes are especially abundant in open recirculating systems due to the concentration of nutrients through the cycling of dissolved solids by evaporation as well as suspended solids being blown in.
In cooling water systems, the wet surfaces are prone to microbiological growth, which in turn can lead to the formation of a biofilm. If left untreated, the biofilm can result in biofouling causing a reduction in plant life and plant efficiency.
Biofouling issues need to be controlled, although the methods of control vary depending on whether the microbes are in a planktonic or sessile state. Sessile microbes are responsible for biofilm formation.
Once biofouling has occurred in a system, even mechanical cleaning cannot remove all traces of a biofilm. Previously fouled surfaces are more susceptible to colonization as residual biofilm materials promote growth and reduce lag time between biofouling episodes. While the effects of biofilm in a system are well known, it is important to keep in mind:Biofouling issues need to be controlled, although the methods of control vary depending on whether the microbes are in a planktonic or sessile state. Sessile microbes are responsible for biofilm formation. Once biofouling has occurred in a system, even mechanical cleaning cannot remove all traces of a biofilm. Previously fouled surfaces are more susceptible to colonization as residual biofilm materials promote growth and reduce lag time between biofouling episodes.
While the effects of biofilm in a system are well known, it is important to keep in mind:
• Biofilms can act as an insulating layer and the performance of the heat exchanger deteriorates in correlation to the thickness of the biofilm
• Microbial Influenced Corrosion (MIC) occurs when the microbes act as catalysts for conventional corrosion
– The presence of the microbes prevents corrosion inhibitors from reaching and passivating the systems metal surfaces
– Corrosion reactions are accelerated by microbiological interactions
– Microbial metabolites can be corrosive to the metalwork
– The most common category of MIC bacteria are sulfate
In this paper, we will investigate the methods in which a large microbiological population in cooling water systems is undesirable and why regular monitoring can minimize the detrimental effects on the system.
Introduction to Microbial Induced Corrosion (MIC)
Corrosion, irreversible deterioration of a metal, is estimated to cost global industry a staggering $2.5Trillion annually.1 The latest figures estimate that 15-35% of that figure could be saved by the implementation of better corrosion controls.
Metal alloys used in the water systems of industrial processes and structures are susceptible to corrosion and the rate and type of corrosion can be determined by several factors. These include:
• Microbiology
• Water Chemistry
• Metallurgy
• Electrochemistry
For the purposes of this paper, the author will concentrate on Microbiology, but the reader should take note that to fully understand and mitigate against corrosion, all four of these elements need to be understood in complex water systems.
Natural waters contain a diverse mixture of microbes, some of which are ubiquitous, and when a metal surface is immersed in water, corrosion and biofilm formation both begin almost immediately.
Corrosion that is caused, or influenced, by microbes is known as Microbial Induced Corrosion – it is also referred to as Microbiologically Influenced Corrosion, Bio-corrosion, or MIC. In literature, it is shown that MIC is not caused by the direct interaction of microorganism with the material but by the reaction of the medium in the area of the biofilm, and in particular from the metabolites of the microbes in question.2
MIC can be observed wherever and whenever an influx of organic substances occurs and suitable surfaces are available for the colonizing of microorganisms that form biofilm. The temperatures of the media play more of a secondary role, as the relevant microorganisms are active within a temperature range of approximately -20°C and 120°C. The ideal temperature, however, is at 10°C to 40°C. Hence, the operational temperature of cooling water systems is within the ideal range that makes them prone to MIC.3
Biofilms
The microbial population in waters can be general divided into two populations:
1. Planktonic (free-floating)
2. Sessile (immobile)
The same species of microorganisms can be found in both populations – Table 1 shows some of the most common microbes found in cooling systems.
In addition to those listed in Table 1, there are many other types of planktonic bacteria in a cooling water system. These planktonic forms come together to form a sessile aggregate that adheres to the inside of the system’s pipework. These sessile bacteria then produce a slime, extracellular polymeric substance, which forms a matrix of DNA, proteins and polysaccharides. The result is a substance that forms a protective layer around the population of bacteria. Biofilm development is normally isolated into three distinct stages of development:
1. Attachment: Bacteria can adhere to surfaces in several ways. This includes variations in metallurgy allowing attachment sites on surfaces, chemical nutrients or a lack of biocide. The primary method of adhesion is through weak Van der Waals forces and hydrophobic effects.
2. Growth: Once initial colonization has occurred, population size increases exponentially. Other bacterial species and inorganic components of the water system contribute to and constitute the complex biofilm layer.
3. Dispersal: Bacteria within the biofilm secrete enzymes and break free of the biofilm itself, becoming planktonic once again. This enables the biofilm to spread to other parts of the water system.
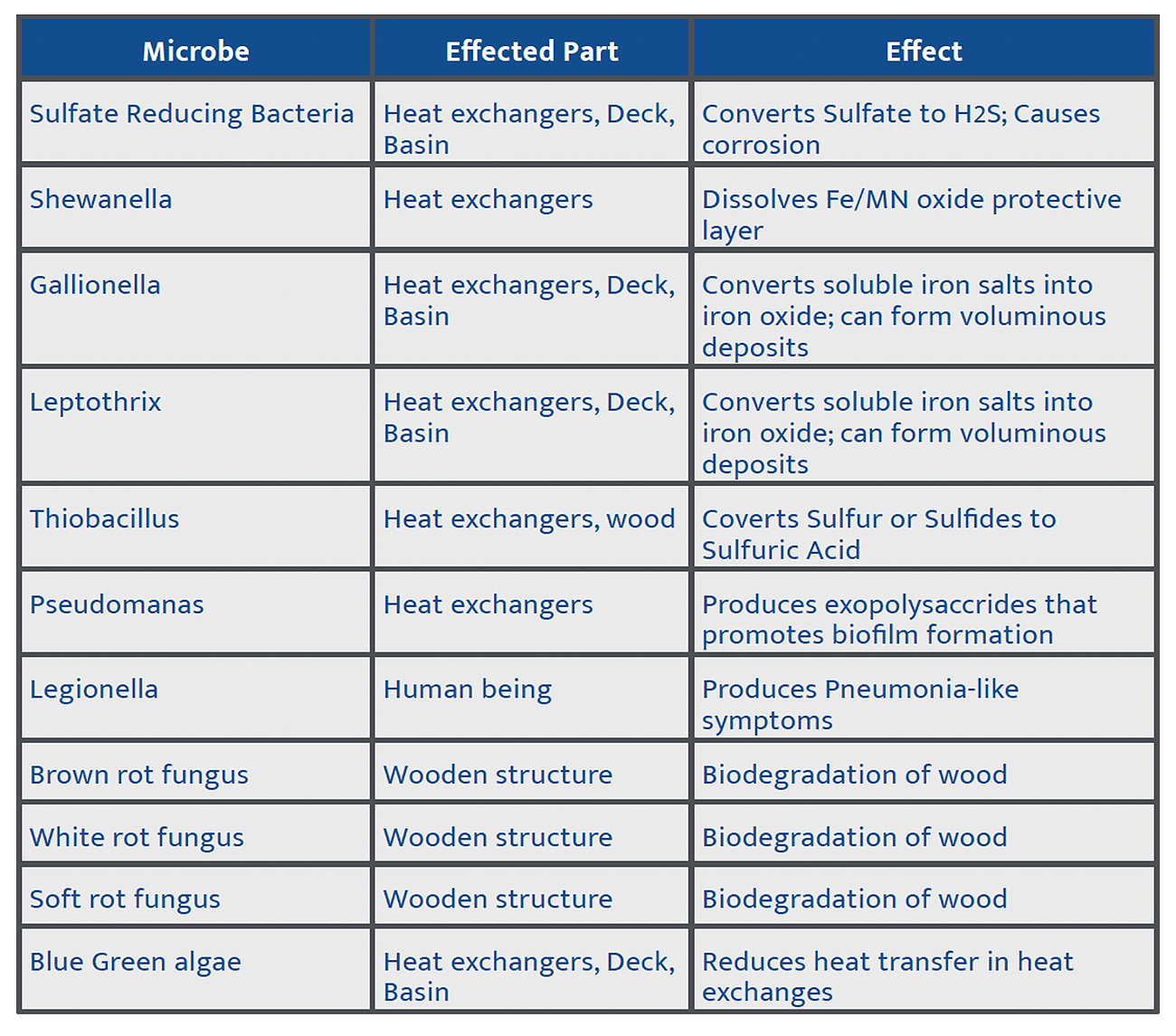
Table 1: Types of microbes affecting cooling towers.
Source: Influence of Microbes in Cooling Tower: A Review; Ranjana Tewari, S K Mehta, Pujan Vaishnav4
Once formed these biofilms can generate aerobic and anaerobic conditions. The anaerobic conditions enable anaerobes such as sulfate reducing bacteria to multiply, and a potential difference is established between different areas of the metal surface.
SRB, such as Desulfovibrio, reduce sulfur compounds like sulfate, sulfite, and thiosulfate to hydrogen sulfide. SRB are thought to be the most prolific contributor to MIC and a major cause of corrosion in open and closed water cooling systems. SRB use several mechanisms of corrosion including cathodic depolarization, anodic depolarization, production of corrosive iron sulfides, sulfide induced stress corrosion cracking and local corrosion.
The presence of SRB is normally indicated by a reddish or yellowish nodule on metal surfaces that, when broken, exhibits black corrosion byproduct. If hydrochloric acid is added to the black deposit, hydrogen sulfide will be released giving off a “rotten egg” odor.
The presence of SRB can contribute to accelerated, localized pitting corrosion and eventual perforation of the pipe. Corrosion by SRB can cause significant damage to surfaces, particularly where pipework may have bends, uneven surfaces, abrasions, or joints and welds. In addition, the amount of nitrite, often used as an anodic corrosion inhibitor in cooling water systems, can be reduced by SRB and therefore in closed heating and chilled systems where nitrite is used SRB must be controlled.
While it is possible to control the MIC process, the corrosion programs that are utilized by water treatment companies are not always effective: Inhibitor levels can drop, be used up and/or provide a source of food for bacteria and other organisms. For example, nitrite-based corrosion inhibitors feeding nitrite reducing bacteria (aka NRBs). It is essential that water quality is monitored, and that microbiological control be at the forefront of any water treatment program. There are several ways in which microbiological control can be achieved, these include:
• Reduction or elimination of suspended solids
• Biocide dosing
• Maintaining water movement throughout the system (elimination of dead legs etc.)
• Monitoring and sampling of the system on a regular basis.
Microbiological analysis of cooling water
A 2016 report estimates that there are approximately 1 trillion species of microbes on Earth with most of those yet to be discovered. 6 When it comes to cooling water, there may be hundreds of species present at any one time, with some of those being very difficult to measure.
It is considered best practice to use “Indicator Organisms” to gain a general overview of the microbiological condition of the water. This translates to: if the level of general bacteria is kept under control, one can assume your system will be kept microbiologically under control.
Traditional methods for these tests have adopted standard agar plate count methods. These are still used in laboratories today and their results are required to fulfil regulatory guidance in many countries. However, the plate count method requires the Operator to: have access to a laboratory; a filtration set up; and training for how to count the results. Because the lab test is cumbersome, an easier method was required for routine monitoring. This is why agar-based dipslides have become so popular.

Figure 1: The left photo shows a cleaned low-carbon steel corrosion coupon with SRB growing on it and producing concentric rings of corrosion. The right photo is a water washed Type 304 stainless steel 5/8 inch condenser tube that has pits and dark spots.4 Source: Understanding and Detecting MIC in Cooling Water Systems; Paul R. Puckorius 5
Dipslides
Agar Dipslides now form part of many legislative guidance papers in terms of routine microbiological monitoring. There are many different types of agar dipslides available for various species. Dipslides are great tools for monitoring for a number of key reasons:
• Relatively inexpensive
• Easy to use – widely considered a “Do-It-Yourself” microbiology test
• Full Results are available within 2-5 days (application dependent).
Because the user has access to the test, checking for early indication (e.g. after 24 hours) is possible
• Allow for trend analysis
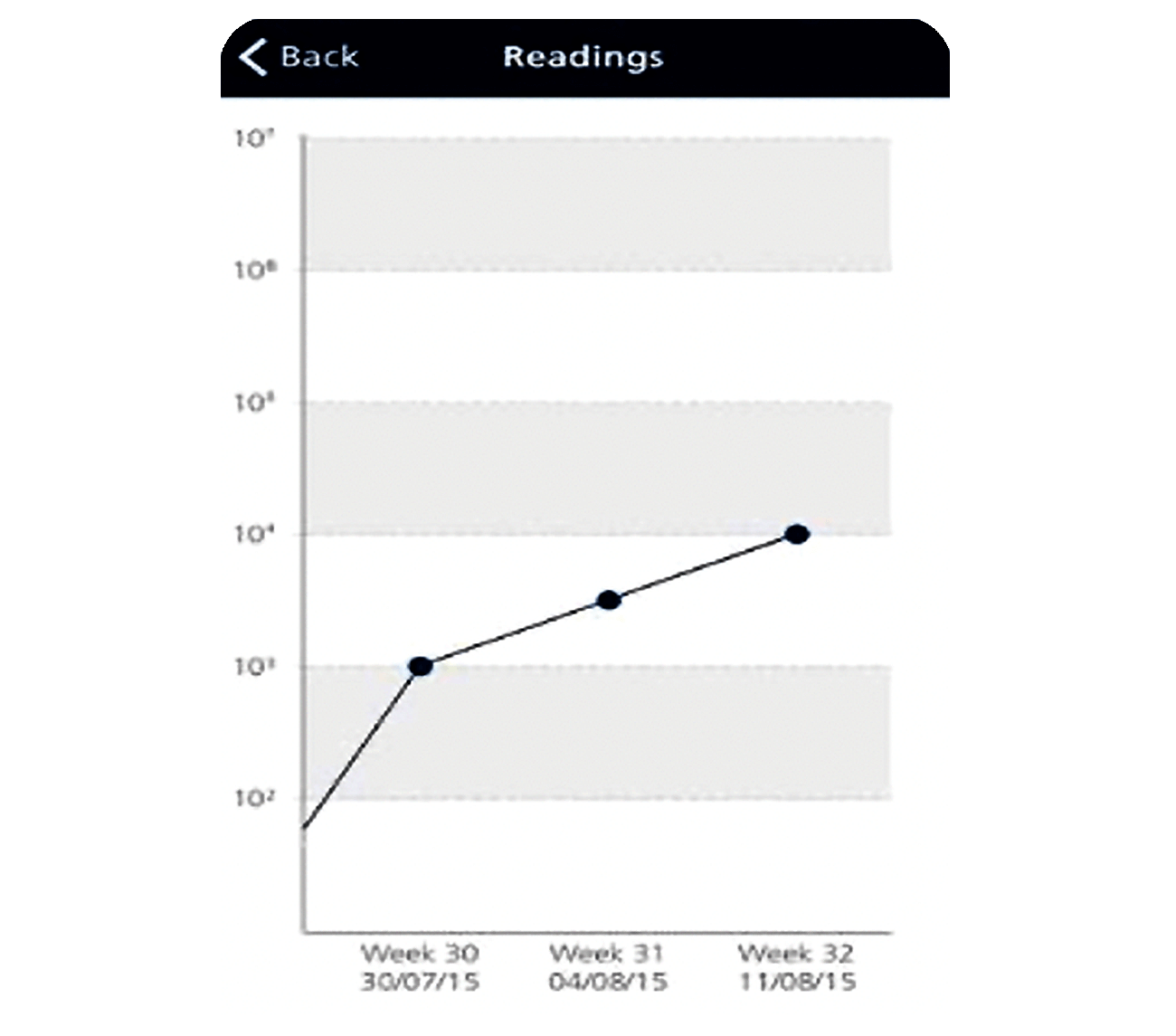
Figure 2: Shows increasing contamination of bacteria on TTC dipslide, demonstrating how trends can be tracked and illustrated.
While there are a lot of advantages to dipslides, users should keep the following in mind:
• Dipslides will only measure the number of Planktonic (culturable) aerobic bacteria in a sample.
• Dipslides will not measure:
– bacteria that will not grow on the type of agar media being used
– bacteria which require a different incubation temperature
– bacteria that are not actively growing at the time of sampling
– bacteria with special growth requirements
– bacteria growing inside biofilms (sessile).
• The correct temperature for incubation forms an integral part of any microbiological test and it is a general misconception that “hotter is better” or “the hotter the incubator, the faster the results”. Neither of these statements are true and in fact, incorrect temperatures can result in misinterpretation of results.
Dipslides should be incubated to best replicate the system from which the sample was taken so that a “true” picture of the general microbiological condition of that system is given. Given the correct conditions, Bacteria can reproduce exponentially, doubling in number in as little as 20 minutes. To help encourage growth, we want to duplicate the environment they are currently surviving as much as possible.
To use humans as an analogy – we are incredibly good at self-regulating our body temperature to 98.6°F (37°C), but our environment can have a direct impact on our ability to maintain that ideal temperature. If a person is simply placed in a desert climate, without proper supplies and protection, they will ultimately end up with heatstroke, which can cause death. Similarly, if you place someone in an arctic climate, hypothermia sets in and will lead to death. A person will not thrive in either condition, because we are not able to regulate our body temperature to an optimum level. This applies to all organisms, including those we are trying to detect in a cooling system.
For example, samples taken from a cooling water sump will have come from an environment where that temperature is approximately 30°C (86°F). The tests methods for testing bacteria from a cooling water are therefore designed to be at the same temperature for optimum results.
The dipslides and agars described below only demonstrate the capability of some of the more popular variants.
Common Types of dipslide available TTC Dipslide
The most common type of dipslide in use is a dual-sided slide that allows convenient enumeration of aerobic microorganisms (TVC or total viable count). The dipslide is prepared with nutrient TTC agar on both sides and is used for general-purpose cultivation of aerobic organisms, which can be obtained from surfaces, fluids or the air. The TTC additive in the nutrient agar reacts with enzymes produced in aerobic respiration to produce a color change from white to red, allowing easy enumeration.
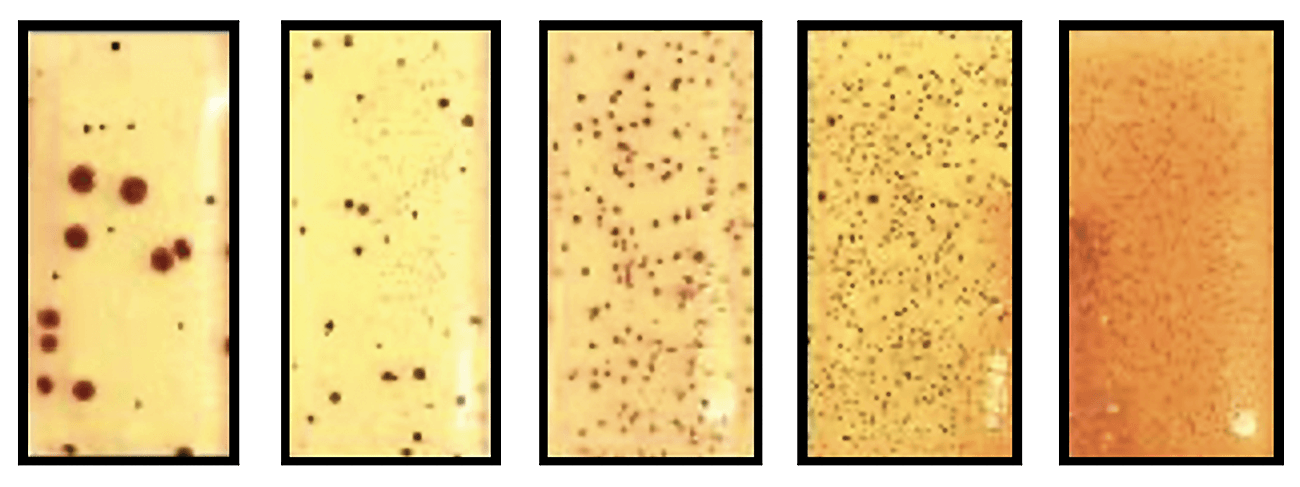
Figure 3: Shows increasing contamination of bacteria on TTC dipslide.
TTC / Rose Dipslide
The TTC/ROSE dipslide allows convenient enumeration of aerobic microorganisms (TVC) including yeasts and molds in a single test. The dipslide is prepared with nutrient TTC agar on one side (responsive to aerobic microbes) and a Rose Bengal agar with chloramphenicol, which selects for yeasts and molds, typically used in the food industry.
The Rose Bengal agar, as its name suggests, gives this side of the slide a distinctive pink coloration, making it easy for the user to identify which side is which. The TTC/ROSE dipslide is also ideal for use in the detection of hydrocarbon utilizing microorganisms found in fuel storage. These microorganisms cause blockages to fuel filters and the acidic metabolites can cause tank erosion. The TTC additive in the nutrient agar reacts with enzymes produced in aerobic respiration to produce a color change from white to red, allowing easy enumeration. The Rose Bengal agar will show growth as white, white/pink or yellow/green spots dependent on the type of yeasts or molds present.
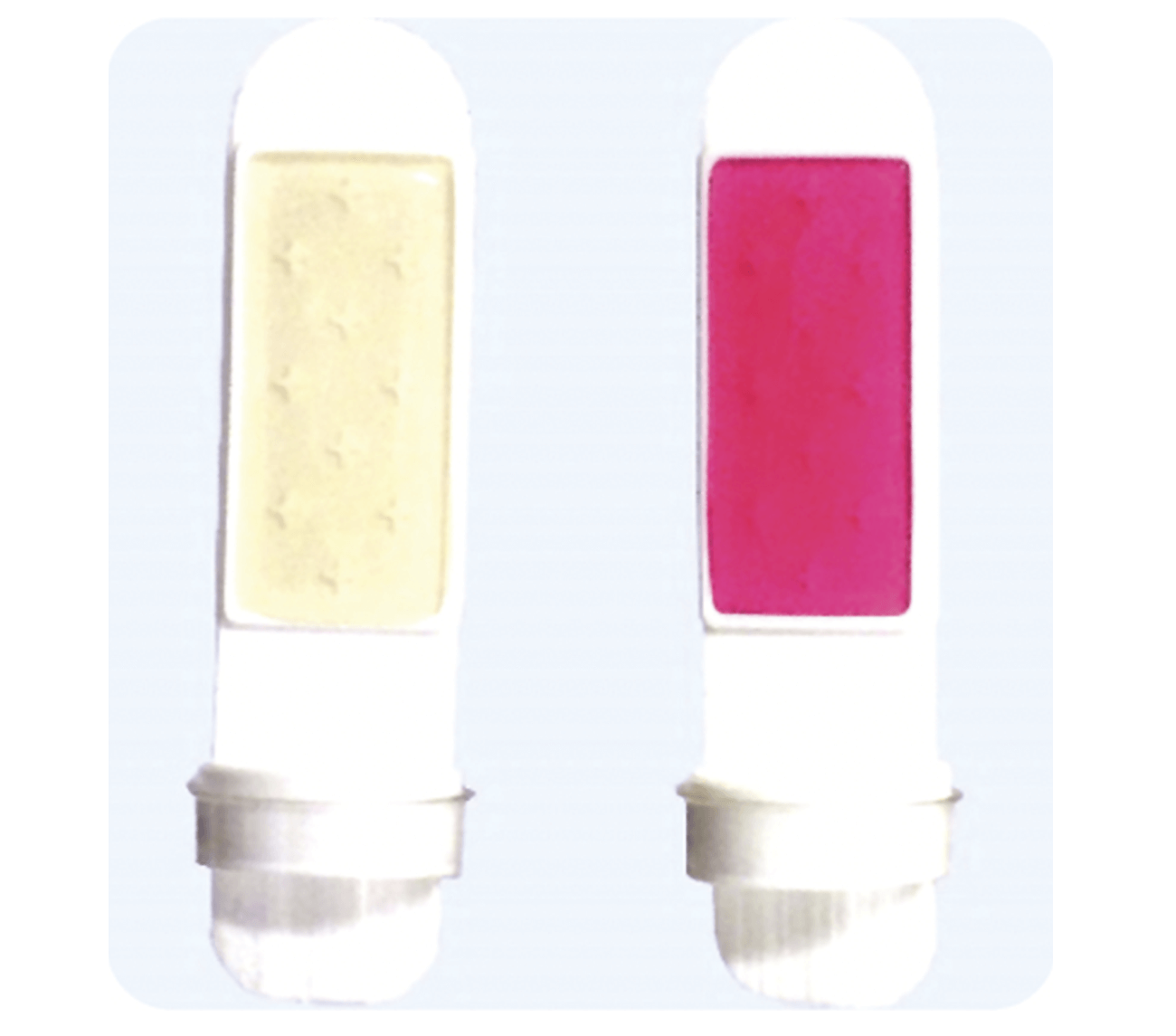
Figure 4: Shows both sides of the TTC/Rose Bengal
Sulfate reducing bacteria (SRB)
A 21-day SRB test is recommended for reference purposes, but shorter tests can provide equal confidence for routine management of the water system provided enough samples are collected and analyzed. The standard method typically uses a modified Postgates broth or modified API RP-38 medium which is inoculated with 1 ml of sample and incubated under anaerobic conditions at 30°C for 21 days.
The shorter tests contain a straw-colored Citrate medium, which reacts to the production of hydrogen sulfide to give a semi-quantitative result after incubation at 35°C for 5 days. The SRB test is used specifically to indicate the presence of bacteria, which under the correct conditions, are able to produce hydrogen sulfide. Hydrogen sulfide is a colorless gas, which is extremely corrosive to ferrous and non-ferrous metals.
Results are determined by the spreading black precipitation from the inoculation point through the agar. When testing samples already high in sulfide there may be a sudden blackening of the medium, in which case, contamination can be judged by the advancement of the blackening further into the medium. The results are semi-quantitative.
The photos below show varying degrees of contamination from a closed circuit water system:
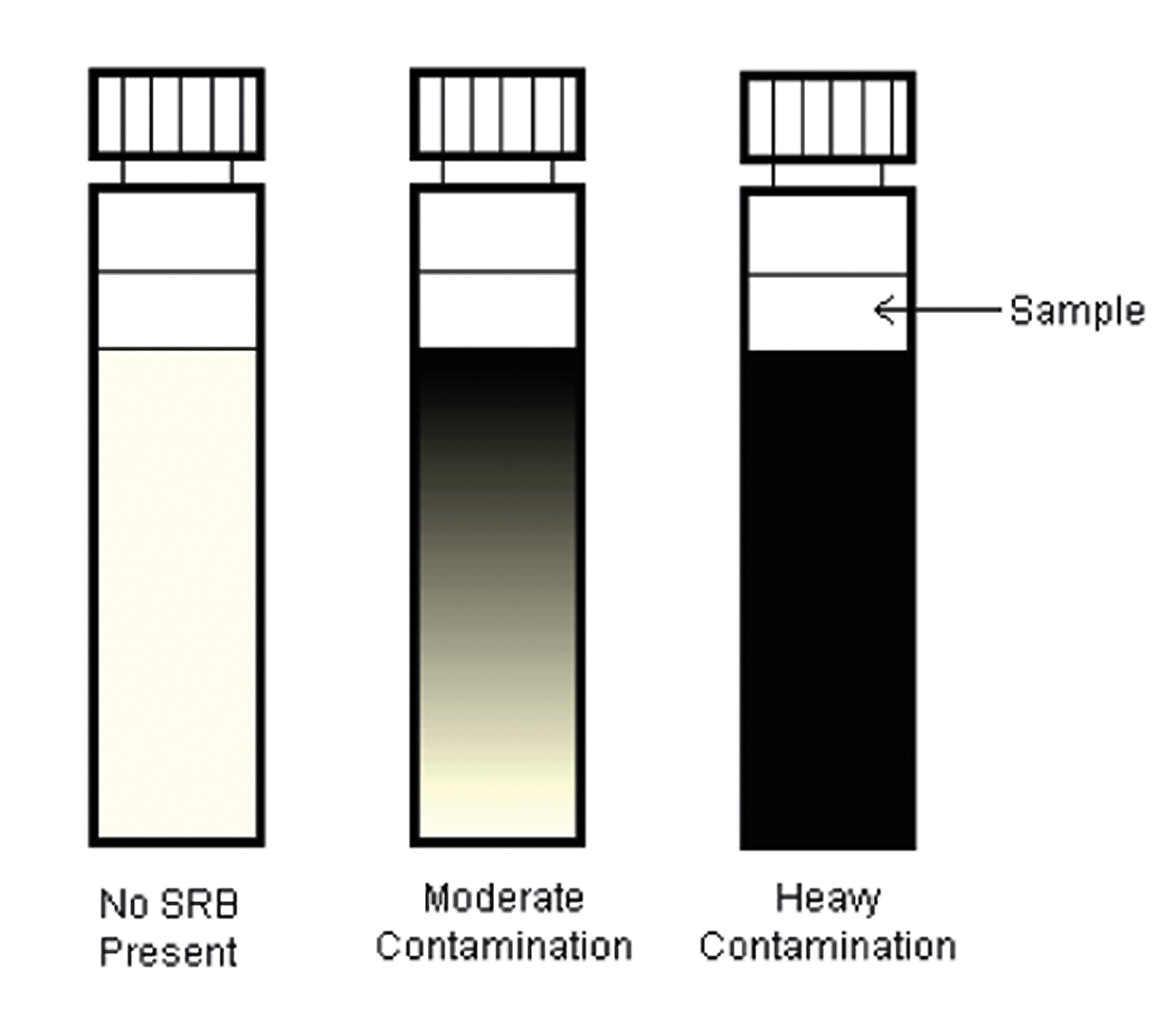
Figure 5: SRB Results Microbiologically Influenced Corrosion. 1st ed 7
Azide spot test
This test is used to confirm the presence of SRB on a metal surface. This test is done on the spot of the corrosion site. A solution of this reagent is placed onto the metal surface for testing. Using a concentrated light and a magnifying lens, nitrogen bubbles are seen to evolve when sulfides are present. The rate of bubble evolution is indicative of the degree of sulfide contamination.
This test is primarily performed in a laboratory and using a microscope for easier visualization.
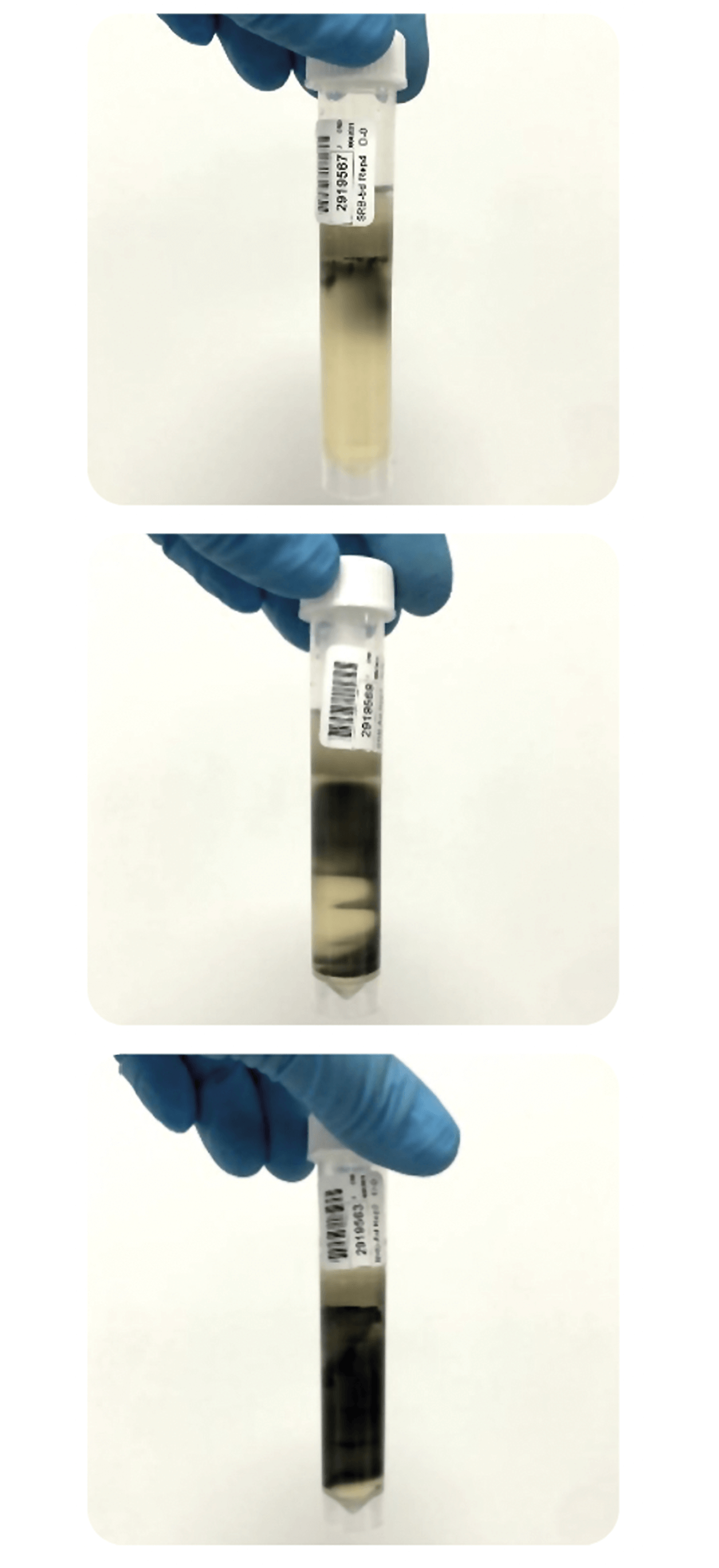
Figure 6: Varying degrees of contamination in SRB 5 day tube test
Source: Authors customer results

Figure 7: High rate of Nitrogen bubble evolution showing, indicating positive indication of SRB
Source: Microbiologically Induced Corrosion; Ibraheem, Ameer K 8
Conclusion
Cooling water systems can be ideal homes for waterborne microorganisms due to their materials of construction, operational temperature, operational pH and a number of other factors. These waterborne organisms contribute to corrosion of the system in their accountability for MIC.
A number of measures can be put in place to control MIC including regular flushing, eliminating dead legs, biocidal treatment, and inhibitors. These preventative actions should all be done in conjunction with regular and consistent monitoring.
Monitoring of indicator organisms is used to determine overall system health, and more specific testing can be done on species particularly associated with MIC – including SRBs.
This monitoring is required at a frequency, which if interpreted correctly, can identify risks quickly allowing remedial action to be taken to minimize any potential damage to the system integrity.
SOURCES
1 Koch, Gerhardus, et al. NACE International, 2016, International Measures of Prevention, Application, and Economics of Corrosion Technologies Study, impact.nace.org/documents/Nace-International-Report.pdf.
2 Heitz, Ewald. Microbially Influenced Corrosion of Materials Scientific and Engineering Aspects; with 56 Tables. 1st ed., Springer, 1996.
3 Bartels, Simone, Richter, Tobias, & Nowak, Erika (2009). Microbially influenced corrosion in cooling water systems Development of a new protection concept for system components conveying brackish water. VGB PowerTech, 89(5), 74-78.
4 Tewari, Ranjana, et al. “Influence of Microbes in Cooling Tower: A Review.” Https://Www.irjet.net/, International Research Journal of Engineering and Technology (IRJET), Aug. 2019, www.irjet.net/archives/V6/i8/IRJET-V6I864.pdf.
5 Puckorius, Paul R. “Understanding and Detecting MIC in Cooling Water Systems.” WATER CONDITIONING & PURIFICATION MAGAZINE, 15 June 2018, wcponline.com/2018/06/15/understanding-detecting-mic-cooling-water-systems/.
6 Bakalar, Nicholas. “Earth May Be Home to a Trillion Species of Microbes.” The New York Times, 23 May 2016, www.nytimes.com/2016/05/24/science/one-trillion-microbes-on-earth.html#:~:text=According%20to%20a%20new%20estimate,the%20number%20of%20insect%20species.
7 Borenstein, Susan. Microbiologically Influenced Corrosion. 1st ed., Springer, 1996.
8 Ibraheem, Ameer K. (2011). Microbiologically Induced Corrosion. 10.13140/RG.2.1.4887.6644.












