As featured in Waterline Summer 2023
The use of chelants / sequestrants in water treatment
Jill Cooper BSc (hons), B&V Chemicals
Chelants such as EDTA and NTA are used widely within water treatment. Their uses include, but are not limited to steam boiler treatment programs, closed circuit pre-commission and flushing chemicals and wastewater treatment programs. Phosphonate and polymer based sequestrants are also widely used and formulated into a diverse range of products including closed circuit inhibitors and cleaners, RO scale inhibitors, cooling tower inhibitors, softener resin cleaners and on-line descaler products for a variety of systems. In this article we will concentrate mainly on the use of chelants and sequestrants in the control and/or removal of metal ions in steam boilers, and closed circuits systems with a particular focus on Fe3+ (ferric iron) and Fe2+ (ferrous iron).
Steam boilers
Metals such as magnesium, copper, iron, and aluminium are commonly found in the feedwater to steam boilers. These metals, and nonmetals including calcium and silica, can produce scale on the metal surfaces of the boiler and associated pipework. Chemical contaminants can settle on top of any initial deposition exacerbating potential problems with flow rates and pressure within pipework as the diameter decreases. Iron contamination from both the feedwater and potentially from boiler condensate and corrosion products from pumps and pipes can deposit on heat exchange surfaces, reducing boiler efficiency and aggravating corrosion in the boiler.
Boiler water dispersants and multipurpose (all in one) steam boiler treatments containing EDTA or NTA will bind to metal ions such as magnesium, calcium, copper, iron, and aluminium. The resulting stable metal chelate is water soluble and ensures that metal ions present in the steam boiler system are captured by the chelant and pass harmlessly through the steam boiler system and out through the blow down. It is worth noting that there should be complete removal of oxygen prior to injection of EDTA or NTA as stated in note 13 of BS2486:1997.
It is also the case that carefully controlled on-line removal of calcium carbonate scale in steam boilers can be achieved using formulations which may contain EDTA, phosphonate and polymer based sequestrants.
The choice of chelant will be dependent on the system requirements. EDTA is an approved raw material as identified in paragraphs (c) and (d) of 21 CFR 173.310. Food and Drug Administration Regulation; Boiler water additives permitted in plants where steam contacts food.
Closed circuit cleaners
Closed circuit cleaners Chelants and sequestrants are often formulated into pre-commission cleaners and flushing chemicals for closed-circuit heating and chiller systems. The other raw materials commonly used in closed circuit cleaner formulations are summarised in the table at the bottom of the page.
The main purpose of pre-commission cleaning and system flushing is to ensure that the pipework is completely clean and to allow the inhibitor to be in contact with the clean metal surface.
The main purpose of pre-commission cleaning and system flushing is to ensure that the pipework is completely clean and to allow the inhibitor to be in contact with the clean metal surface.
In the case of pre-commission cleaners, a chelant based formulation should only be needed if corrosion within the system has occurred prior to pre-commission cleaning. There should be no corrosion / insignificant levels of corrosion if:
• The system has been designed to limit ingress of oxygen and pipework has been stored and installed correctly.
• Initial fill of a system for pressure testing includes the addition of an inhibitor and biocide to the system.
• The system after initial fill has been circulated regularly.
• The time between first fill and pre-commission cleaning is short i.e., weeks not months.
If the system has been treated as described in the above paragraph, and no significant corrosion has occurred, a gentler cleaner without a chelant should clean the system sufficiently. Most of these gentler cleaners will contain a sequestrant such as a phosphonate which should be sufficient to remove any mill scale present. Mill scale (a type of iron oxide formed during hot rolling) is bluish black in colour, usually less than 0.1 mm (0.0039 in) thick and adheres to the steel surface and protects it from atmospheric corrosion, provided no break occurs in this coating.
On-line cleaner CPC cleaning procedures
A closed loop pre-treatment cleaning (CPC) procedure for filling and cleaning systems, ensures that they are filled with an appropriate closed-circuit scale and corrosion inhibitor and biocide from first fill. This approach should be the case for all conventional and CPC cleaning methods in new closed systems. With CPC cleaning the system is recirculated and cleaned through a combined process of velocity and filtration. The procedure is thus quicker and results in a less aggressive clean of pipework than traditional methods, also ensuring that no water or minimal water is discharged to drain.
However, CPC cleaning is not suitable for pre-commission cleaning of every system. Some closed circuits are filled with water for pressure testing and allowed to sit and stagnate for long periods, by which time corrosion and biofilm may be an issue. In these cases, conventional pre-commission cleaning with more aggressive chelant based cleaners is likely to be required.
Chelant based cleaners
Choosing the right chelant based formulation is essential to maximise the effectiveness of the cleaning process. It is also critical to understand that chelants are effective under different conditions, which can affect their ability to remove corrosion deposits at various pHs.
Both EDTA and NTA are used widely in pre-commission cleaners and system flush chemicals. These formulations will also generally contain sequestrants, polymers and surfactants. Most products will also be formulated around neutral or at slightly acidic pH i.e., 4.5 – 7.5.
One advantage of using NTA in formulations is due to its stability at a lower pH than EDTA. Closed circuit cleaners containing NTA can therefore be formulated at pH 4.5 – 5.5. Any formulation containing EDTA must generally be formulated over pH 7.5 to ensure stability of the formulation.
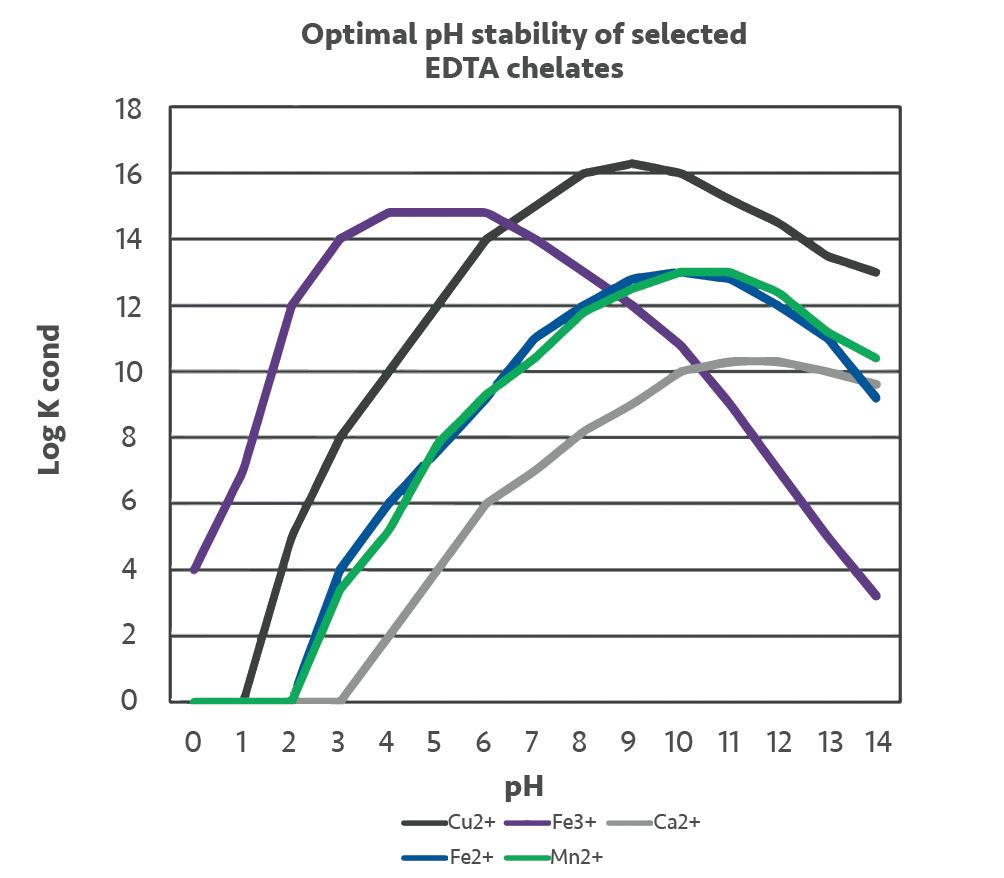
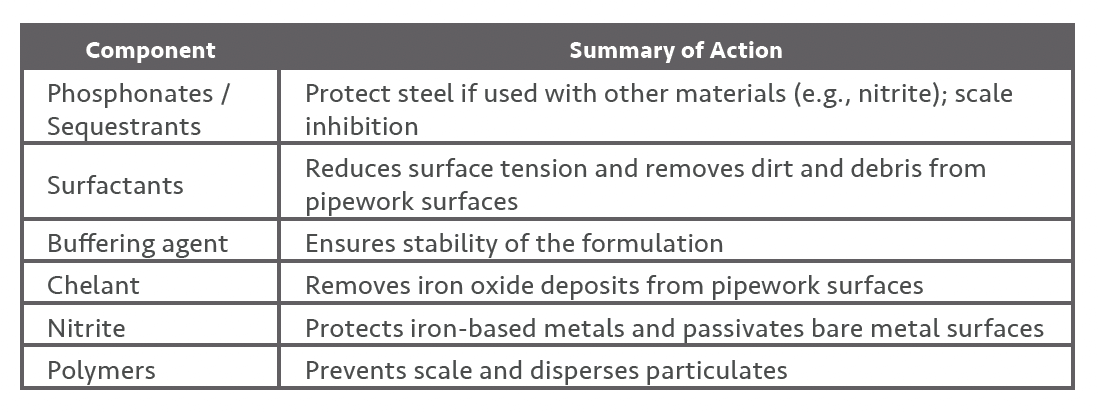
The graphs illustrate the optimal pH for NTA and EDTA for specific metal ions i.e., these graphs illustrate the fact that the pH of the recirculating solution is critical for the removal of iron-based corrosion deposits.
Both NTA and EDTA will both work well in the chelation of ferrous iron (Fe2+) and ferric iron (Fe3+) but have very different optimal pH’s for iron saturation.
Although the most effective pH for the chelation of ferric iron (Fe III) by EDTA is between 3-6, chelation will still occur over the range of 2-10. For NTA, the optimal pH is around 3 although chelation will still occur at pHs up to around 8. This needs to be considered when iron saturation limits / iron plateau levels for specific formulations are being calculated.
Most formulated pre-commission cleaners and flushing agents will also contain a phosphonate which may also chelate / sequester iron; typically, the optimal pH for these phosphonates to work in chelating iron is around pH 10. The differing efficiency at which iron is chelated by different agents under various conditions makes calculating the maximum iron saturation levels for any closed-circuit cleaner containing multiple chelant/sequesterants very complicated.
As cleaning progresses and iron oxide (corrosion) is removed from the pipework surface the pH of the solution will naturally rise. The benefit of NTA based formulations is that the starting pH of the formulations will generally be around pH 5 in comparison with the EDTA based formulations which typically start at a pH of around 7.5. Any NTA based formulation will therefore be able to chelate iron between pH 5 up to pH 8 whereas EDTA formulations can chelate iron well up to pH 10.
Examples
Calculations indicate that our main EDTA formulation, based on the EDTA content alone, can chelate 1200ppm Fe3+ at 1%. The formulation also contains other sequestrants, which further enhances the chelating power of the product. At lower pHs, a formulation containing NTA is likely to perform better. 1% of our main NTA containing formulation can theoretically collate 4000ppm Fe3+ based on its NTA content. It must be noted that other factors will affect these values including the presence of other metal ions in solution with pH being a critical factor.
If the pH rises above pH 10 for EDTA based cleaners or pH 8 for NTA based cleaners the pH in the system will need to be reduced. This reduction in pH can be achieved by adding more cleaner OR by adding carefully controlled amounts of a suitably formulated acidic product.
For pHs in the optimum range, once the iron level in the system has plateaued, it is generally recommended that more cleaner is added, and the system allowed to circulate for a minimum of another hour and the iron levels re-tested. If iron levels remain at the same level after an hour, the system should be allowed to circulate for a further hour and then re-tested. Three comparable iron levels would generally indicate that an iron plateau level has been reached.
It is important to bear in mind that chelants will also bind, copper, calcium, magnesium etc. and that the speed of the chemical cleaning process will be affected by temperature and whether the iron is in solution. BSRIA BG29 states that a chemical clean can take anything between 12 – 72 hours so sufficient site time should be allocated to ensure a thorough clean can be achieved.

Lab trials showing how different products have different efficacies at pulling iron into solution from a corroded coupon.
The above is just a snapshot of some applications where chelants can be used; there are many more within the field of water treatment.












